Katherine A. Freitas, Julia A. Belk, Elena Sotillo, Patrick J. Quinn, Maria C. Ramello, Meena Malipatlolla, Bence Daniel, Katalin Sandor, Dorota Klysz, Jeremy Bjelajac, Peng Xu, Kylie A. Burdsall, Victor Tieu, Vandon T. Duong,Micah G. Donovan, Evan W. Weber, Howard Y. Chang, Robbie G. Majzner, Joaquin M. Espinosa, Ansuman T. Satpathy, Crystal L. Mackall
Science, 11 November 2022
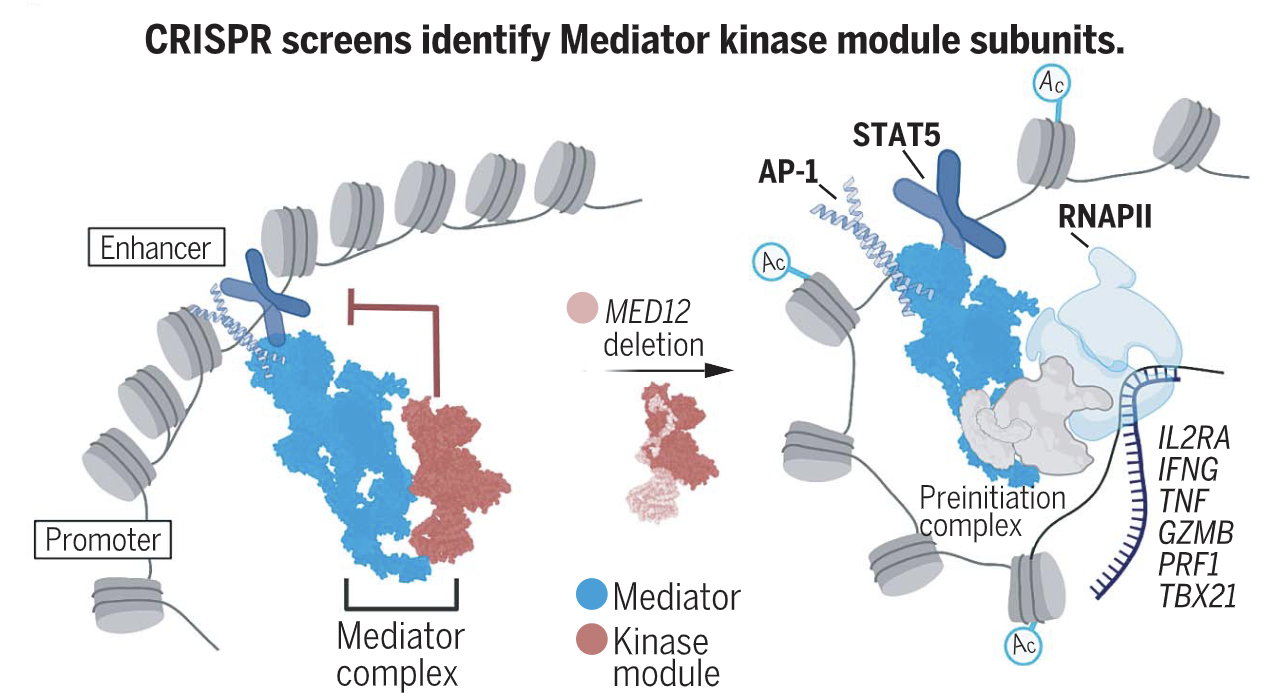
T cells are the major arm of the immune system responsible for controlling and regressing cancers. To identify genes limiting T cell function, we conducted genome-wide CRISPR knockout screens in human chimeric antigen receptor (CAR) T cells. Top hits were MED12 and CCNC, components of the Mediator kinase module. Targeted MED12 deletion enhanced antitumor activity and sustained the effector phenotype in CAR- and T cell receptor–engineered T cells, and inhibition of CDK8/19 kinase activity increased expansion of nonengineered T cells. MED12-deficient T cells manifested increased core Meditator chromatin occupancy at transcriptionally active enhancers—most notably for STAT and AP-1 transcription factors—and increased IL2RA expression and interleukin-2 sensitivity. These results implicate Mediator in T cell effector programming and identify the kinase module as a target for enhancing potency of antitumor T cell responses.