BenceDaniel, Julia A.Belk, Stefanie L.Meier, Andy Y.Chen, Katalin Sandor, ZsoltCzimmerer, ZsofiaVarga, Krisztian Bene, Frank A.Buquicchio, YanyanQi, Hugo Kitano, Joshua R. Wheeler, Deshka S. Foster, Michael Januszyk, Michael T. Longaker, Howard Y. Chang, Ansuman T. Satpathy
Molecular Cell, 14 December 2022
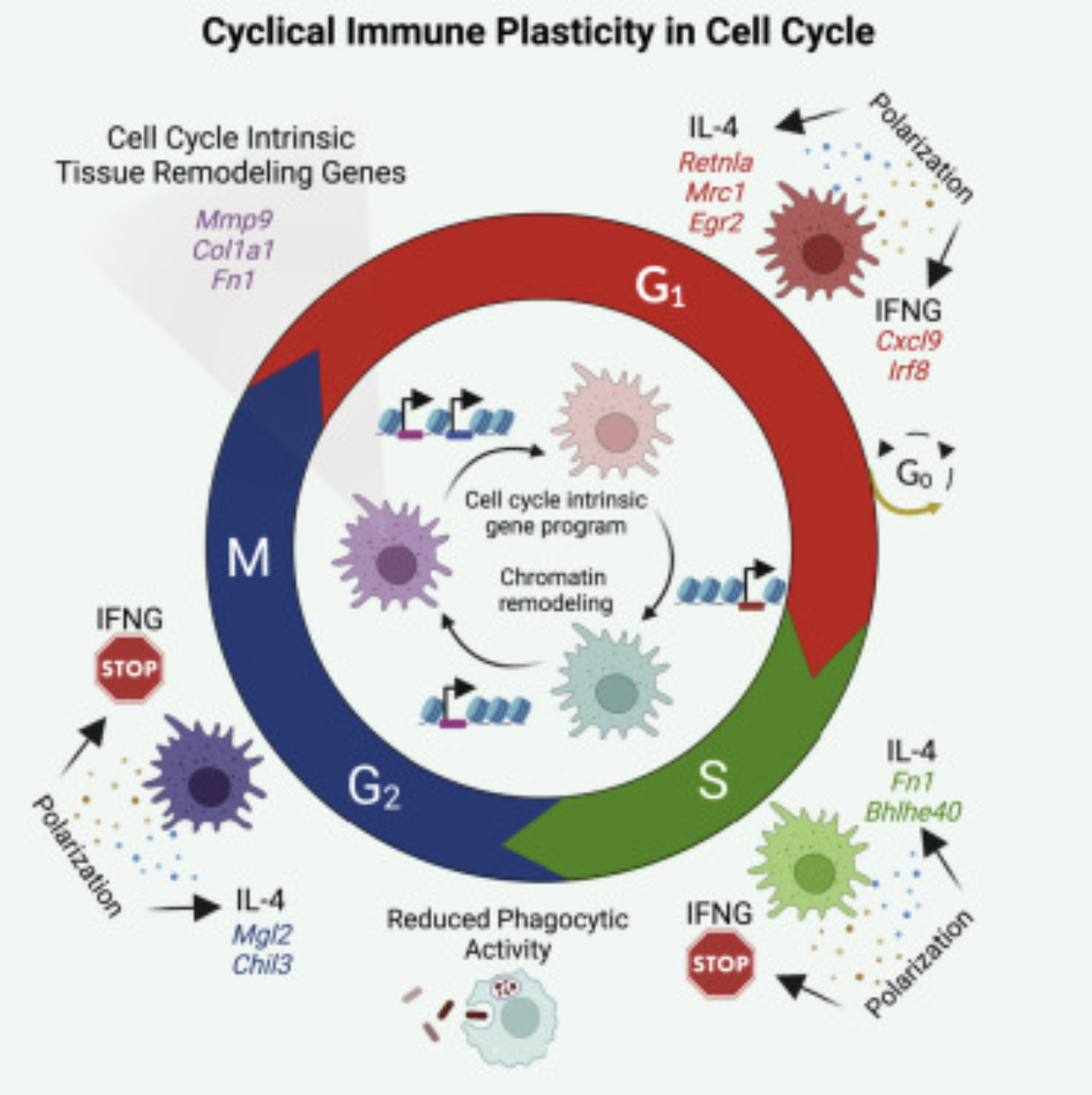
Cell cycle (CC) facilitates cell division via robust, cyclical gene expression. Protective immunity requires the expansion of pathogen-responsive cell types, but whether CC confers unique gene expression programs that direct the subsequent immunological response remains unclear. Here, we demonstrate that single macrophages (MFs) adopt different plasticity states in CC, which leads to heterogeneous cytokine-induced polarization, priming, and repolarization programs. Specifically, MF plasticity to interferon gamma (IFNG) is substantially reduced during S-G2/M, whereas interleukin 4 (IL-4) induces S-G2/M-biased gene expression, mediated by CC-biased enhancers. Additionally, IL-4 polarization shifts the CC-phase distribution of MFs toward the G2/M phase, providing a subpopulation-specific mechanism for IL-4-induced, dampened IFNG responsiveness. Finally, we demonstrate CC-dependent MF responses in murine and human disease settings in vivo, including Th2-driven airway inflammation and pulmonary fibrosis, where MFs express an S-G2/M-biased tissue remodeling gene program. Therefore, MF inflammatory and regenerative responses are gated by CC in a cyclical, phase-dependent manner.