Satpathy AT*, Saligrama N*, Buenrostro JD*, Wei Y, Wu B, Rubin AJ, Granja JM, Li R, Mumbach MR, Lareau CA, Serratelli WS, Gennert DG, Schep AN, Corces MR, Kim YH, Khavari PA, Greenleaf WJ, Davis MM*, Chang HY*. *contributed equally
Nature Medicine, 1 May 2018
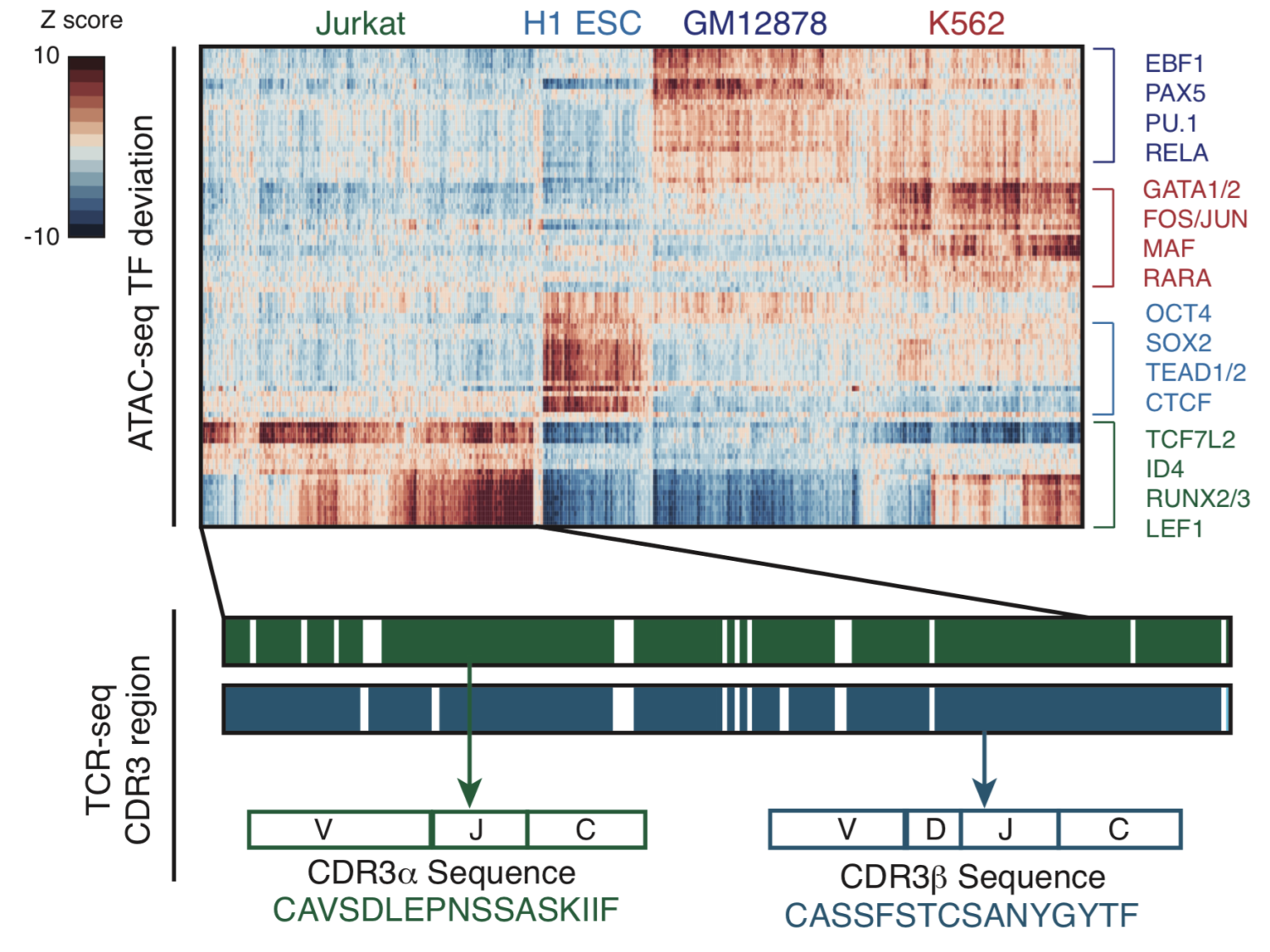
T cells create vast amounts of diversity in the genes that encode their T cell receptors (TCRs), which enables individual clones to recognize specific peptide-major histocompatibility complex (MHC) ligands. Here we combined sequencing of the TCR-encoding genes with assay for transposase-accessible chromatin with sequencing (ATAC-seq) analysis at the single-cell level to provide information on the TCR specificity and epigenomic state of individual T cells. By using this approach, termed transcript-indexed ATAC-seq (T-ATAC-seq), we identified epigenomic signatures in immortalized leukemic T cells, primary human T cells from healthy volunteers and primary leukemic T cells from patient samples. In peripheral blood CD4+ T cells from healthy individuals, we identified cis and trans regulators of naive and memory T cell states and found substantial heterogeneity in surface-marker-defined T cell populations. In patients with a leukemic form of cutaneous T cell lymphoma, T-ATAC-seq enabled identification of leukemic and nonleukemic regulatory pathways in T cells from the same individual by allowing separation of the signals that arose from the malignant clone from the background T cell noise. Thus, T-ATAC-seq is a new tool that enables analysis of epigenomic landscapes in clonal T cells and should be valuable for studies of T cell malignancy, immunity and immunotherapy.